How do you solve a problem like ammonia monitoring?
Published: 13 June 2024
One of the many challenges associated with delivering Section 82 of the Environment Act is ammonia monitoring. Section 82 requires water companies to continuously monitor the quality of water in watercourses upstream and downstream of an asset outfall for a number of parameters including ammonia.

We have to remind ourselves why ammonia has been listed within the Environment Act as a target measurement. It is simply that ammonia in a water body can cause stress, gill, and internal organ damage, and eventually death to the fish. This is more so when pH levels are greater than pH8.0. There are currently two methods used for measuring or inferring ammonia levels in solution.
Selective Ion Electrodes SEI have been used extensively for many years in identifying water quality, although the ones used for ammonia typically only detect ammonium. Ammonium is much less toxic because it cannot cross the cell membranes of animals. Ammonia however can, and that's why ammonia is dangerous to fish. Therefore, SEI technology is limited to only inferring potential harm when pH exceeds 8.0. The chemical equation that drives the relationship between ammonia and ammonium is: NH 3 + H2O <-> NH 4 + + OH – and is further explained as a percentage in the table produced by Emerson, Ruso and Lund.
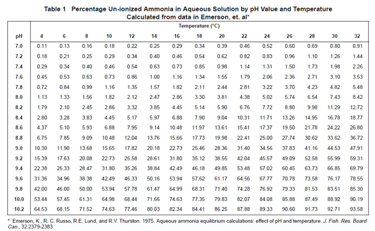
The big question currently doing the rounds in parliament and the Environment Audit Commission is, with ammonia being so dangerous to fish, should we discourage any form of inference and focus purely on measuring ammonia directly?
Wet chemistry and colorimetric analysis ‘Spectrometry’ are still the most common method used for quantifying aqueous ammonia in the laboratory. CFR 40 Chapter I Subchapter D Part.
136 explains the standards used within this method; salicylate method and Nessler method or the two most common methods used.
Salicylate method has the advantage over Nessler as it avoids the use of toxic reagents. The salicylate method is one of the available colorimetric ammonia quantification methods based on the Berthelot reaction. The term “Berthelot reaction” refers to a family of chemical reactions where a phenolic compound reacts with ammonia and a hypochlorite source to form an indophenol-like dye. A colorimeter ‘Optical Engine’ is then used to identify the amount of ammonia present in the solution.
However, with any optical engine / Spectrometry, its great in controlled conditions in a laboratory, but when used in the field, there can be associated problems with fouling of the lenses and in certain cases turbidity may affect the light intensity. Although monitoring ammonia in gaseous form, light intensity is less important.
The search goes on for a method to accurately measure ammonia in the river, but time is running out.
Read more in our latest article on the challenges of delivering Section 82 here.
More from our Knowledge Hub
 Case study
Case studyGroup companies Detectronic and Oneline combine to deliver hydraulic improvements at Meole Brace Golf Course
 Insights
InsightsA prevention-first future: Our response to the government’s white paper, 'A New Vision for Water'
 Insights
InsightsFrom insight to impact: Delivering a solution to pollution at WWT Wastewater 2026
 Case study
Case studySafeguarding data centre continuity through strategic DSEAR risk assessment
Environmental compliance today, creating a sustainable tomorrow
Helping you reduce risk to the environment and your operation by managing assets compliantly while achieving commercial, ESG, and net-zero goals.
Contact our experts
